Introduction to Aquarium pH

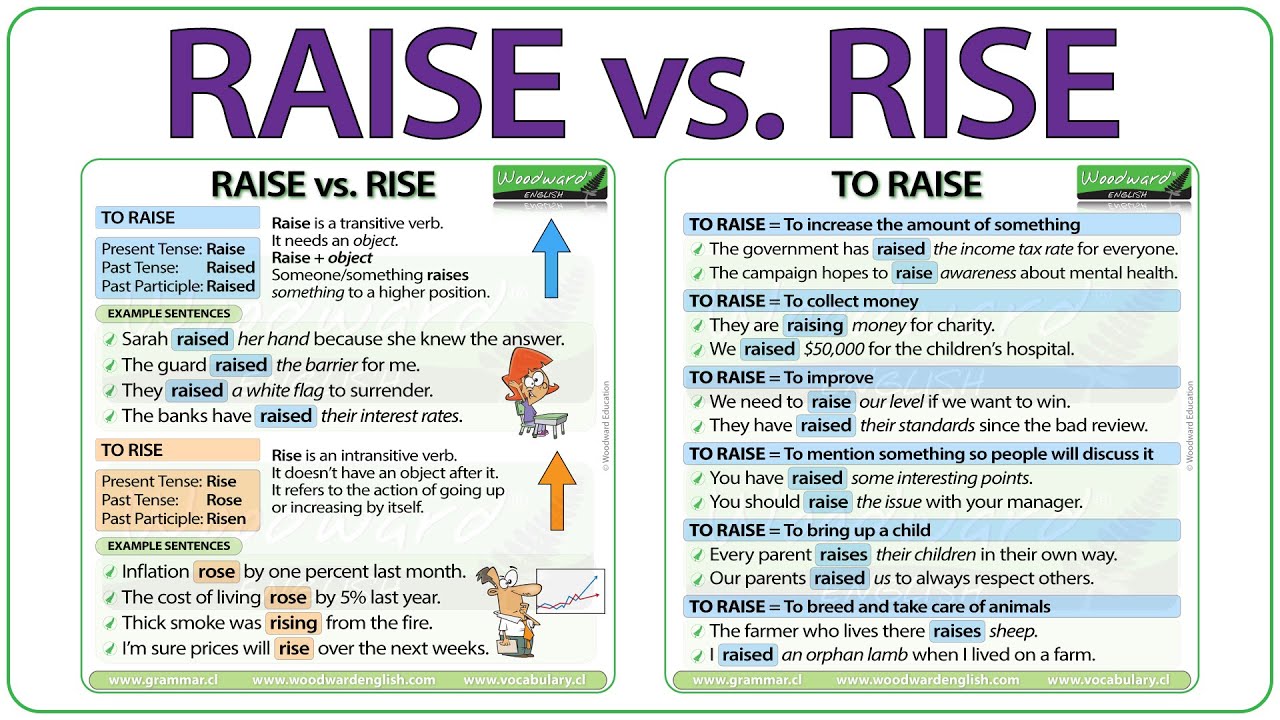
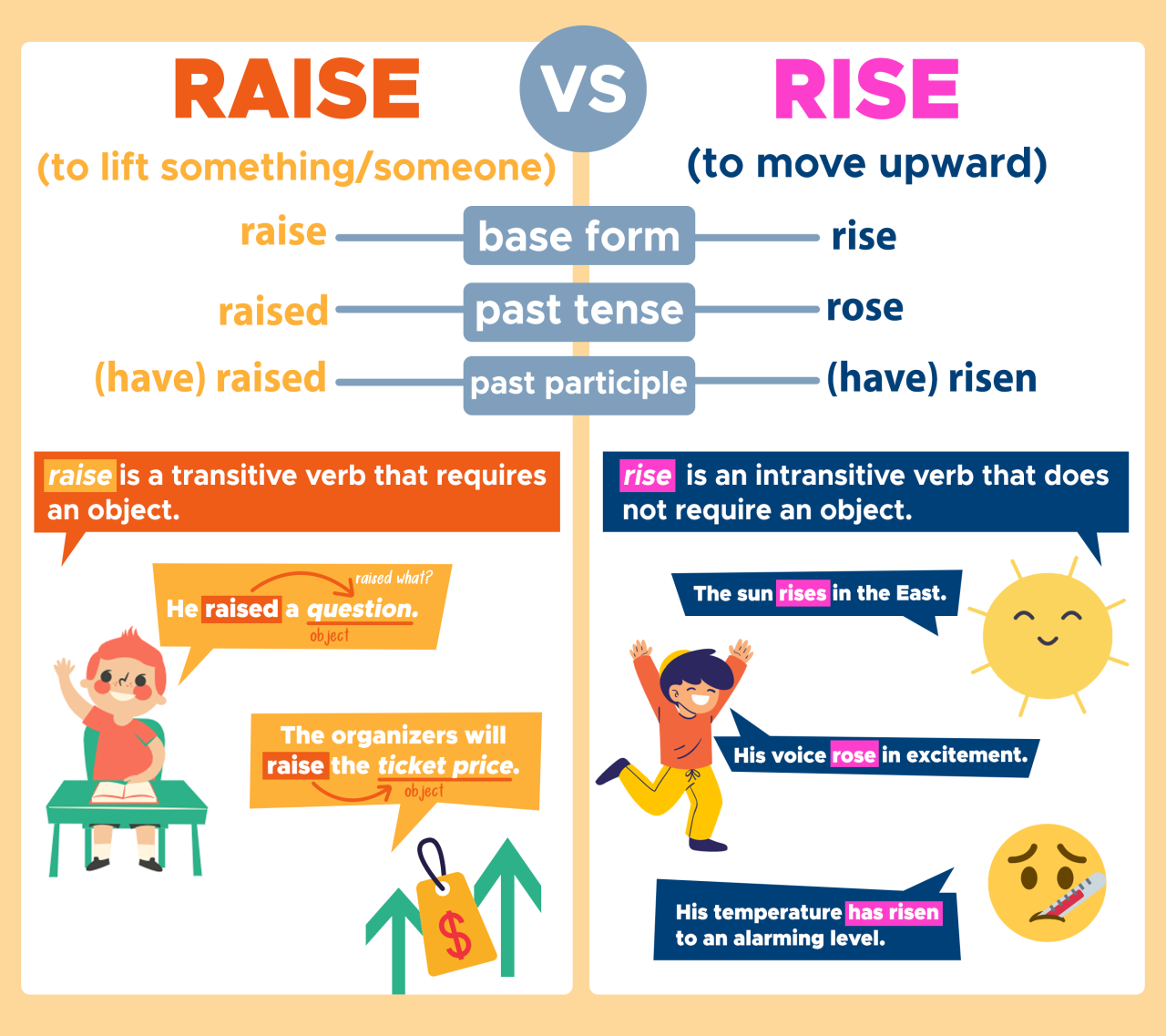
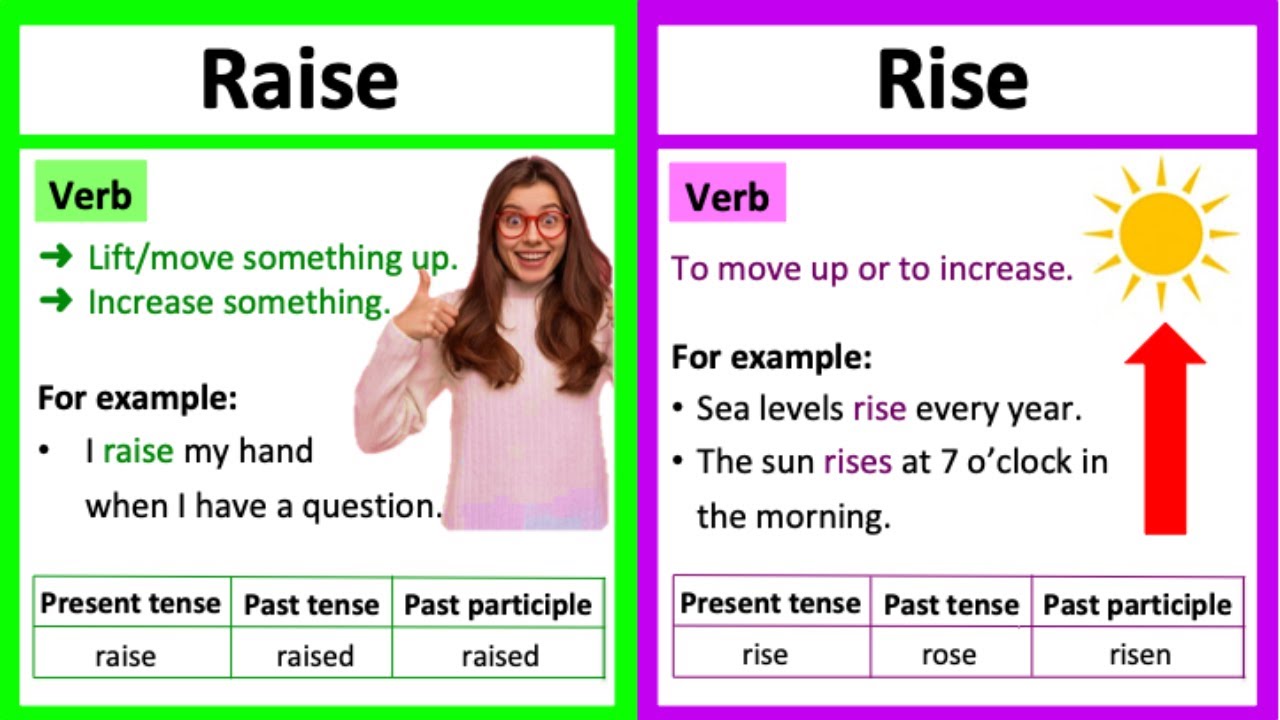
Aquarium pH, or potential hydrogen, is a measure of the acidity or alkalinity of the water. It’s a crucial factor in maintaining a healthy and thriving aquatic environment. Understanding pH and its importance is fundamental to successful fishkeeping. A stable pH level directly impacts the overall well-being of your fish.
Maintaining a consistent pH level is vital for a balanced aquarium ecosystem. Variations in pH can cause stress, hinder proper nutrient uptake, and ultimately affect the fish’s health. This can manifest in various ways, impacting their growth, reproduction, and overall survival.
Ideal pH Ranges for Different Fish Species
Different fish species have specific pH preferences. Providing the appropriate pH range mimics their natural habitats, promoting optimal health and well-being. It’s important to research the specific needs of your fish to ensure a suitable environment.
- Cichlids typically thrive in slightly alkaline water with a pH range of 7.0 to 8.5. This range closely resembles the pH levels found in their native habitats, supporting their natural behaviors and biological processes.
- Betta fish, also known as Siamese fighting fish, prefer a slightly acidic to neutral pH, typically between 6.5 and 7.5. Maintaining this range will support their well-being and promote optimal behavior.
- Goldfish are generally adaptable and can tolerate a wider range of pH levels. While they are not as sensitive to pH variations as some other species, maintaining a pH range of 6.5 to 7.5 is ideal for their overall health and longevity.
Common Symptoms of pH Imbalances, How to raise ph in aquarium
Variations in pH can lead to several observable symptoms in your fish. Understanding these indicators can help you promptly address any issues and restore a healthy balance.
- Loss of Appetite: A sudden or prolonged lack of appetite can be a sign of stress or discomfort, potentially linked to pH fluctuations. This can affect their growth and overall health.
- Fin and Body Rot: Changes in pH can weaken the fish’s immune system, making them susceptible to infections. Fin and body rot are common signs of compromised health and can be aggravated by fluctuating pH levels.
- Lethargy and Unusual Swimming Patterns: Fish exhibiting lethargy or erratic swimming behavior might be experiencing stress from pH variations. Observing these changes is crucial for timely intervention.
- Loss of Color: Some fish species exhibit color changes when the pH is out of balance. This is often a sign of stress and a potential indicator of a need to adjust the pH level.
pH Measurement and Testing
Accurate pH measurement is essential for maintaining optimal aquarium conditions. Regular testing allows for proactive management and timely adjustments to prevent issues.
Regular monitoring of the pH level is crucial to maintain a stable environment. This can be achieved by using test kits specifically designed for aquarium water. These kits are readily available and provide accurate readings, enabling you to monitor pH changes.
Causes of Low Aquarium pH

Aquarium pH levels are crucial for maintaining a healthy environment for fish and other aquatic life. A low pH can indicate underlying issues that need immediate attention to prevent stress and potential health problems in the aquarium inhabitants. Understanding the factors that contribute to low pH is essential for proactive aquarium management.
Maintaining a stable pH is vital for the well-being of aquatic life. Different species have varying pH preferences, and deviations from the optimal range can trigger stress responses and compromise their overall health. Monitoring and adjusting pH levels proactively helps to create a more hospitable and balanced environment for the diverse life forms in the aquarium.
Water Source Characteristics
Water sources vary significantly in their inherent chemical compositions, which directly influence the pH of the aquarium water. The mineral content, particularly the presence of carbonates and bicarbonates, plays a pivotal role. Hard water, rich in these minerals, tends to have a higher pH, whereas soft water, with lower mineral content, typically results in a lower pH. Understanding the pH of your water source is a crucial initial step in establishing an appropriate aquarium environment. For instance, well water or rainwater often have lower pH values compared to municipal tap water.
Substrate Choices
The substrate, the material at the bottom of the aquarium, can also influence the pH. Certain substrates, like peat-based materials, release organic acids into the water, leading to a decrease in pH. This effect is often temporary, and the rate of pH change depends on the type and quantity of peat used. Other substrates, such as gravel or sand, generally have minimal impact on pH, but the presence of certain minerals in the substrate can still influence the overall water chemistry.
Decaying Organic Matter
The accumulation of decaying organic matter, such as uneaten food, fish waste, and dead plants, contributes significantly to lowering the pH in aquariums. The decomposition of these materials releases acids into the water, thereby decreasing the pH. Regular water changes and effective filtration are essential for preventing the buildup of decaying organic matter and maintaining a healthy pH. Overfeeding fish is a common cause of decaying organic matter, leading to rapid and significant pH drops.
Common Causes
- Water Source: Using water with naturally low pH levels, such as rainwater or well water, will result in a lower aquarium pH. This is often the primary cause for new aquariums.
- Substrate Choice: Using substrates containing peat or other organic materials can lead to a gradual decrease in pH. This is a slower but significant factor that needs consideration.
- Overfeeding: Feeding fish more than they can consume leads to excess uneaten food and fish waste, accelerating the decomposition process and reducing pH.
- Inadequate Filtration: Insufficient filtration allows the buildup of organic waste, further contributing to lower pH levels.
- Lack of Water Changes: Regular water changes help remove accumulated waste products, thus preventing the pH from dropping.
Methods to Raise Aquarium pH

Raising the pH in an aquarium is a crucial aspect of maintaining a healthy environment for your aquatic inhabitants. Understanding the various methods and their potential impacts is vital for success. Improperly managed pH adjustments can lead to stress and illness in fish, highlighting the importance of a cautious and informed approach.
Different methods offer varying degrees of control and effectiveness. Some are quicker and more direct, while others offer a more gradual and potentially less stressful adjustment. Selecting the appropriate method hinges on factors like the extent of pH adjustment needed, the size of the aquarium, and the specific needs of the fish species.
Chemical Buffering Agents
Chemical buffers are often employed to raise aquarium pH. These substances work by resisting changes in pH, effectively maintaining a stable level. Various types of chemical buffers are available, each with its unique characteristics. The selection depends on the desired level of control and the specific needs of the fish.
Substrate Changes
Substrate alterations can influence aquarium pH. Different substrates possess varying buffering capacities, affecting the overall pH level. Choosing a substrate that promotes the desired pH level can be advantageous, though it requires careful consideration of the fish species’ compatibility with the new substrate type.
pH Up Products
Numerous pH up products are commercially available, providing a convenient method for raising aquarium pH. These products are formulated to introduce alkaline compounds into the water, directly increasing the pH. It’s important to carefully evaluate the product’s composition, as some may contain harmful chemicals.
Comparison of pH Up Products
| Product Name | Effectiveness | Cost | Safety |
|---|---|---|---|
| Aquarium pH Up | High | Medium | High (if used as directed) |
| pH Buffer | Moderate | Low | Moderate (check ingredient list) |
| Alkaline Substrate | Low to Moderate (dependent on substrate) | Low | High |
Note: Effectiveness ratings are subjective and depend on factors like water chemistry and product concentration. Cost is relative to the amount needed for a given aquarium size. Safety is based on the product’s composition and proper usage. Always refer to product labels for specific instructions.
Risks of Rapid pH Changes
Rapid shifts in aquarium pH can induce significant stress on aquatic inhabitants. Fish, invertebrates, and plants may experience various adverse reactions, including loss of appetite, lethargy, and, in severe cases, death. Gradual adjustments are crucial for minimizing these risks. Observing the aquarium’s inhabitants closely for any signs of distress is vital during and after any pH adjustment. A gradual, measured approach minimizes stress and promotes the well-being of the aquarium ecosystem.
Substrate and Water Changes: How To Raise Ph In Aquarium
Maintaining the ideal pH in your aquarium requires a multifaceted approach. Substrate selection and regular water changes play crucial roles in achieving and maintaining the desired pH level. These practices are integral to the health and well-being of your aquatic inhabitants.
Understanding how substrate choices and water changes impact pH is essential for aquarium hobbyists. This section details the intricacies of these procedures, offering a step-by-step guide for effective water changes that minimize pH fluctuations. Furthermore, it explores the relationship between substrate types and their buffering capabilities, crucial for long-term pH stability.
Substrate Selection and pH Influence
Aquarium substrates, while often overlooked, can significantly impact pH levels. Different substrates possess varying buffering capacities, influencing how the pH responds to external factors. This inherent capacity to resist changes in pH is a critical factor to consider when selecting a substrate.
- Certain substrates, like crushed coral or certain gravel mixes, have inherent buffering properties, helping to maintain a more stable pH. These materials can absorb or release ions, thereby mitigating fluctuations.
- Other substrates, such as certain types of sand, may not have significant buffering capacity. In these cases, pH fluctuations are more pronounced and require more proactive management, such as frequent water changes or pH adjustments.
Water Changes and Their Impact on pH
Regular water changes are fundamental to maintaining a healthy aquarium environment. These changes remove accumulated waste products, replenish essential minerals, and dilute substances that can affect pH.
- Performing water changes gradually is essential. A sudden large-scale water change can induce a significant pH shift, potentially stressing or harming your fish.
- The frequency and volume of water changes depend on factors such as the size of the aquarium, the stocking density, and the type of filtration system employed.
Step-by-Step Guide for Gradual Water Changes
A gradual approach to water changes is vital to avoid abrupt pH shifts. This strategy minimizes stress on the aquatic inhabitants and helps maintain a stable environment.
- Prepare the replacement water: Allow the replacement water to reach room temperature, ensuring it matches the temperature of the aquarium water. This step prevents shock to the fish.
- Calculate the water change volume: Determine the appropriate amount of water to replace based on the aquarium size and guidelines for your fish species. This is a crucial factor for the appropriate amount of change and the level of stability.
- Slowly introduce the replacement water: Introduce the prepared water gradually, over a period of several hours. This ensures a smooth transition and prevents drastic pH changes.
- Monitor pH levels: Regularly monitor the pH levels of the aquarium water throughout and after the water change. Adjust the amount of water changed if necessary to achieve the desired pH levels.
Examples of Substrates and Their pH Buffering Properties
The pH buffering properties of substrates vary significantly. Choosing the right substrate is crucial for maintaining a stable pH in your aquarium.
| Substrate | pH Buffering Properties | Typical pH Range |
|---|---|---|
| Crushed coral | High | 8.0 – 8.4 |
| Gravel (certain types) | Moderate | 6.5 – 7.5 |
| Sand (certain types) | Low | 6.0 – 7.0 |
Frequency of Water Changes for Optimal pH
The optimal frequency of water changes depends on several factors. Consistency and careful monitoring are key to success.
- For heavily stocked aquariums, more frequent water changes are usually required. This is due to the increased bioload and the greater rate of waste production.
- A general guideline is to perform partial water changes weekly, replacing approximately 10-20% of the water each time.
Biological Filtration and pH

Biological filtration plays a crucial role in maintaining a healthy and stable aquarium environment, directly influencing the pH level. A well-functioning biological filter promotes a balanced ecosystem where beneficial bacteria thrive, impacting the chemical makeup of the water, including pH.
The presence and activity of these beneficial bacteria are pivotal in regulating the aquarium’s pH. They break down harmful ammonia and nitrite, products of fish waste and uneaten food, into less toxic substances, like nitrate. This breakdown process, while critical for water quality, can also slightly affect the pH. Understanding these interactions is essential for maintaining a stable and optimal pH for your aquatic inhabitants.
The Role of Beneficial Bacteria
Beneficial bacteria, specifically nitrifying bacteria, are essential for converting toxic ammonia and nitrite into less harmful nitrate. These bacteria colonize the filter media, creating a biological filtration system. This process, called nitrification, involves two stages: ammonia oxidation to nitrite, and nitrite oxidation to nitrate. The presence and activity of these bacteria directly impact pH.
Effect of Beneficial Bacteria on pH Levels
The nitrification process, while crucial for water quality, can slightly alter the pH. The oxidation of ammonia to nitrite often results in a slight decrease in pH. However, the overall effect depends on several factors including the specific bacteria species, the amount of ammonia being processed, and the buffering capacity of the aquarium water. Generally, the pH fluctuations are often subtle and manageable with proper filtration and water changes.
Ammonia, Nitrite, and Nitrate Levels and pH
The levels of ammonia, nitrite, and nitrate directly influence the aquarium’s pH. High ammonia and nitrite levels can severely impact pH and harm fish. These compounds are acidic, and their presence in excess can cause a significant drop in pH. Conversely, high nitrate levels generally have a less drastic impact on pH but can still contribute to imbalances. Maintaining optimal levels of ammonia, nitrite, and nitrate through proper filtration is crucial for a stable pH.
Maintaining a Healthy Biological Filter
Maintaining a healthy biological filter is essential for stable pH levels. Regular cleaning of the filter media is necessary to prevent the buildup of waste products and to ensure the beneficial bacteria continue to thrive. However, excessive cleaning can disrupt the bacterial colony, impacting pH. A balanced approach is key, ensuring the filter is adequately cleaned without completely removing the beneficial bacteria. Providing a consistent source of food for fish and ensuring proper waste removal, as well as frequent water changes, supports the filter’s effectiveness and stability of the pH.
Connection Between Proper Filtration and pH Stability
Proper filtration, including regular maintenance and sufficient capacity, is vital for maintaining a stable pH. A healthy biological filter efficiently converts harmful ammonia and nitrite into less toxic nitrate, minimizing the impact of these substances on the pH. Furthermore, the stable filtration system reduces the frequency of water changes needed to maintain pH, which further contributes to stability. A consistent, healthy filtration system is essential to support a stable pH, and the overall health of the aquarium ecosystem.
Testing and Monitoring Aquarium pH

Maintaining the ideal pH level in your aquarium is crucial for the health and well-being of your aquatic inhabitants. Regular testing and monitoring allows you to proactively address any pH fluctuations and prevent potential stress or disease. Understanding how to accurately test and interpret results is essential for effective aquarium management.
Importance of Regular pH Testing
Regular pH testing is vital for ensuring the well-being of your fish and other aquatic life. Fluctuations in pH can cause stress, weaken immune systems, and make fish more susceptible to disease. A stable pH level is crucial for proper metabolic functions and the effective functioning of the aquarium’s biological filter. Detecting and correcting pH issues early minimizes risks to your fish and provides an optimal environment.
Step-by-Step Procedure for Accurate pH Testing
Accurate pH testing requires a methodical approach. Follow these steps to obtain reliable readings:
- Gather the necessary materials, including the chosen pH testing kit or meter, deionized or distilled water (for calibration if needed), and clean test tubes or containers.
- Prepare the test solution by carefully following the manufacturer’s instructions for your specific testing method. This often involves carefully measuring a sample of aquarium water into the appropriate container. Pay attention to the volume specifications.
- Add the reagents provided in the kit or introduce the water sample to the meter’s sensor. Ensure you adhere to the exact procedures detailed in the instructions to guarantee precision.
- Observe the color change or digital reading, meticulously comparing it to the color chart or the meter’s display. Note that the accuracy of color matching is crucial, and slight variations can influence the result.
- Record the pH reading and the date and time of the test in a logbook or digital document. Consistent record-keeping is important for tracking trends and identifying patterns.
Various pH Testing Methods
Several methods are available for testing aquarium pH. Choosing the appropriate method depends on your budget, desired accuracy, and personal preference.
- Test Kits: These kits provide a visual comparison using color charts or liquid indicators. They are generally affordable and convenient for regular home use. Examples include liquid drop test kits, where a reagent is added to the water sample, and the resulting color is compared to a color chart. The accuracy of these kits can vary depending on the brand and the quality of the reagents.
- Digital pH Meters: These meters provide a digital reading of the pH level. They are generally more accurate than test kits, but they require calibration and maintenance. Digital meters often offer precise readings and can be used to monitor pH over time. Calibration ensures the meter’s accuracy by using a standard solution to adjust its reading.
Interpreting pH Test Results
Understanding the significance of pH readings is crucial. A pH reading of 7.0 is considered neutral. Readings below 7.0 are acidic, and above 7.0 are alkaline. The ideal pH range for most tropical fish is typically between 6.5 and 7.5. Readings outside this range can indicate potential issues and require corrective action. Consult your fish’s specific needs to determine the optimal pH.
Frequency of pH Monitoring
The frequency of pH monitoring depends on several factors, including the stability of your aquarium’s water parameters, the type of fish you keep, and any recent changes to the aquarium environment. For newly established aquariums or those undergoing significant water changes, more frequent monitoring is advisable, such as daily checks. Once stable, weekly testing can be sufficient. Monitoring allows you to anticipate potential problems and adjust accordingly.
Gradual pH Adjustments
Maintaining a stable and healthy aquarium environment requires careful attention to various parameters, including pH. Sudden changes in pH can be detrimental to the fish, causing stress and potentially leading to illness or death. A gradual approach to adjusting pH levels is crucial for the well-being of aquatic life.
Gradual pH adjustments are essential to minimize stress on the fish. Fish are remarkably sensitive to changes in their environment. Rapid shifts in pH can disrupt their internal balance, leading to various physiological issues. A gradual approach allows fish to adapt to the changing conditions, minimizing stress and maximizing their chances of survival.
Importance of Gradual Adjustments
Sudden shifts in pH can induce a range of adverse effects on fish. Stress, loss of appetite, and erratic behavior are common responses. More severe consequences include gill damage, internal organ malfunction, and even death. A gradual approach, on the other hand, allows the fish to acclimate, minimizing the risk of these negative impacts. A steady increase in pH, monitored carefully, allows the fish to adjust their internal processes to the new conditions.
Methods for Gradual pH Adjustments
A systematic approach to raising pH involves several key steps. Regular partial water changes are essential, allowing for a slow and controlled increase in the desired pH level.
- Partial Water Changes: Replacing a portion of the aquarium water with water of the desired pH level is a crucial component of a gradual adjustment. This method allows for a slow and controlled increase in pH, giving the fish time to adapt to the new conditions. The amount of water changed should be limited to 10-20% of the total aquarium water volume in a single water change. This allows the fish to acclimate to the new pH level and ensures the beneficial bacteria in the aquarium are not severely affected. For example, if the aquarium contains 100 liters of water, replacing 10-20 liters with water having the desired pH level in each change is a good practice.
- pH Up Additives: Commercial pH up additives can be used to gradually raise the pH level. However, it’s crucial to follow the manufacturer’s instructions carefully and add the product slowly. Excessive use can cause sudden and detrimental pH fluctuations. Begin by adding small amounts of the additive, monitoring the pH levels regularly and adjusting as needed. For example, start with a quarter of the recommended dose and observe the results before increasing the amount.
- Substrate and Water Changes: Adjustments to the substrate and frequent water changes, as described previously, can help maintain a consistent and gradually increasing pH. The type of substrate used can influence the pH of the water, and regular water changes help maintain a consistent environment. A gradual shift in the pH level allows the beneficial bacteria to adapt, thus minimizing any disruption to the biological filtration system.
Effects of Sudden pH Changes
Sudden pH changes can have significant negative impacts on fish health. Fish have a complex internal balance, and rapid pH shifts can disrupt this balance. Examples include increased susceptibility to diseases, loss of appetite, and erratic behavior. This can lead to a cascade of negative consequences, including death.
Safe and Effective Methods
Using a combination of gradual water changes and pH up additives, carefully monitored, provides a safe and effective approach to raise pH levels. Regular testing and monitoring are essential for tracking progress and ensuring that the changes are gradual and manageable. By following the suggested methods, fish can adapt to the new pH levels with minimal stress.
pH and Aquarium Plants
Aquarium plants, like their terrestrial counterparts, thrive within a specific pH range. Understanding this range and how to maintain it is crucial for successful plant growth and overall aquarium health. Proper pH management directly impacts nutrient availability and plant metabolism, influencing the vibrant aesthetic and vitality of your aquatic garden.
Effect of pH on Plant Growth
Plant growth in aquariums is intricately linked to pH levels. Different plant species have varying optimal pH requirements. A pH that’s too high or too low can hinder nutrient uptake, resulting in stunted growth, discoloration, and even plant death. Maintaining the appropriate pH level allows plants to absorb essential nutrients effectively, fostering robust growth and vibrant coloration.
Adjusting pH for Specific Plant Types
Different aquatic plant species have specific pH preferences. Some plants thrive in slightly acidic conditions, while others flourish in more alkaline environments. Researching the specific pH range for each plant species you intend to cultivate is essential. For instance, certain species of Anubias are known to prefer a slightly acidic environment, while others, such as some varieties of Amazon Swords, might prefer a more neutral to slightly alkaline pH.
Role of Nutrients and pH in Plant Health
Nutrient availability is directly affected by pH. Certain nutrients are more readily available at specific pH levels. For example, iron availability is often optimal in slightly acidic conditions. Maintaining a balanced pH allows for optimal nutrient absorption, promoting healthy growth and vibrant coloration.
Maintaining a Balanced Environment for Plants and Fish
Creating a harmonious environment for both plants and fish is vital. While specific pH levels are beneficial for plant growth, it’s also essential to consider the fish species you keep in the aquarium. Many fish species have a preferred pH range that overlaps with the optimal pH for specific plant types. Researching the pH requirements of both plants and fish ensures a balanced environment for all inhabitants.
Examples of Plants Thriving in Different pH Ranges
| Plant Type | Optimal pH Range | Description |
|---|---|---|
| Anubias | 5.5 – 7.0 | These stem plants are generally adaptable to slightly acidic conditions. |
| Amazon Swords | 6.0 – 7.5 | These popular stem plants generally prefer a neutral to slightly alkaline pH. |
| Java Fern | 6.0 – 7.5 | Java ferns are highly adaptable and can tolerate a wide range of pH conditions. |
| Water Lettuce | 6.5 – 7.5 | Water lettuce is a fast-growing, floating plant that thrives in a wider pH range. |
These examples highlight the diverse pH preferences among aquarium plants. Further research into specific plant varieties can provide a more precise understanding of their optimal pH requirements. This tailored approach ensures the health and beauty of your aquarium ecosystem.
Troubleshooting Low pH Issues

Maintaining a stable and optimal pH level in an aquarium is crucial for the health and well-being of aquatic inhabitants. Fluctuations or consistently low pH can lead to stress, disease susceptibility, and even mortality. Identifying the root cause of low pH and implementing appropriate corrective measures are vital for maintaining a thriving aquarium environment.
Addressing low pH issues effectively requires a systematic approach that involves diagnosing the problem, understanding the potential contributing factors, and implementing suitable solutions. This process often involves careful observation, regular testing, and making adjustments to the aquarium’s parameters.
Common Low pH Problems
Identifying the source of low pH is often the first step in resolving the issue. Several factors can contribute to persistently low pH levels. Common problems include improper water source, inadequate filtration, and poor water changes. Understanding these issues will allow for more effective solutions.
Solutions for Specific Low pH Issues
A systematic approach to resolving low pH problems requires understanding the root cause. For example, if the issue is linked to the water source, the solution involves using a water conditioner or adjusting the water source itself. If the problem is due to insufficient water changes, regular and adequate water changes can help to restore the desired pH levels.
Diagnosing and Resolving Low pH Problems
A systematic approach to diagnosing and resolving low pH problems involves several key steps. First, regularly test the aquarium water parameters, including pH, ammonia, nitrite, and nitrate levels. Second, carefully observe the fish and other inhabitants for any signs of stress, such as loss of appetite, lethargy, or unusual behavior. Third, identify the potential contributing factors to the low pH.
Example Scenarios and Solutions
Several scenarios illustrate the importance of a systematic approach to resolving low pH issues.
- Scenario 1: Low pH due to a hard water source. If your tap water has a high hardness level, it can contribute to low pH in the aquarium. In this case, using a water conditioner to lower the hardness of the water or using a different water source can effectively solve the problem.
- Scenario 2: Insufficient water changes. If water changes are infrequent or inadequate, accumulated waste products can lower the pH. Increasing the frequency and volume of water changes, alongside the addition of a high-quality filter media, can restore the desired pH.
- Scenario 3: Overfeeding. Overfeeding can cause a buildup of organic waste, potentially lowering the pH. Adjusting feeding schedules and ensuring that only the food that is consumed within a reasonable timeframe is provided can be effective. Regular cleaning of the aquarium substrate is also crucial.
Diagnosing and Solving Low pH Issues in Your Aquarium
To effectively diagnose and solve low pH issues in your aquarium, regularly test your water parameters and note any changes in fish behavior. Look for patterns and correlations between these observations and potential contributing factors. For example, a sudden drop in pH could be related to a recent water change using untreated water. Alternatively, a gradual decrease in pH might be linked to overfeeding or inadequate filtration.
Record the results of your tests and any observations about your aquarium inhabitants’ behavior to track trends. This will allow for the identification of patterns and the potential cause of the low pH. Implementing a systematic approach to diagnosis and correction, combined with regular monitoring, is crucial for maintaining a healthy and stable aquarium environment.
Answers to Common Questions
How to raise ph in aquarium – What are the common symptoms of pH imbalance in fish?
Symptoms can include loss of appetite, lethargy, erratic swimming, fin and body damage, and even death. It’s crucial to address any observed issues promptly.
How often should I test the pH in my aquarium?
Regular testing, ideally weekly, is recommended. More frequent checks may be necessary during initial pH adjustments or if you notice unusual behavior or water quality changes.
Can I use any type of substrate to raise the pH?
No, not all substrates are suitable. Some materials, like certain types of gravel or sand, have pH-buffering properties that can help maintain a stable pH. Research different substrate types to find one appropriate for your specific fish and desired pH.
What are some natural ways to adjust pH in my aquarium?
Besides chemical buffers, you can use substrate changes, which help to increase the pH gradually. Water changes are also important for maintaining optimal pH levels.